Why You Should be Concerned if Your Cycle is Not Working Properly
”Methylation happens over a billion times a second. It is like one big dance, with biochemicals passing methyl groups from one partner to another” (Braly, The H Factor Solution).
This is the most scientific chapter of this book and also one of the most important chapters. Please take your time and slowly read through to understand how methylation impacts virtually every aspect of your health. I want you to truly understand why methylation is so critical and why you should be concerned if you are not supporting this pathway in your body.
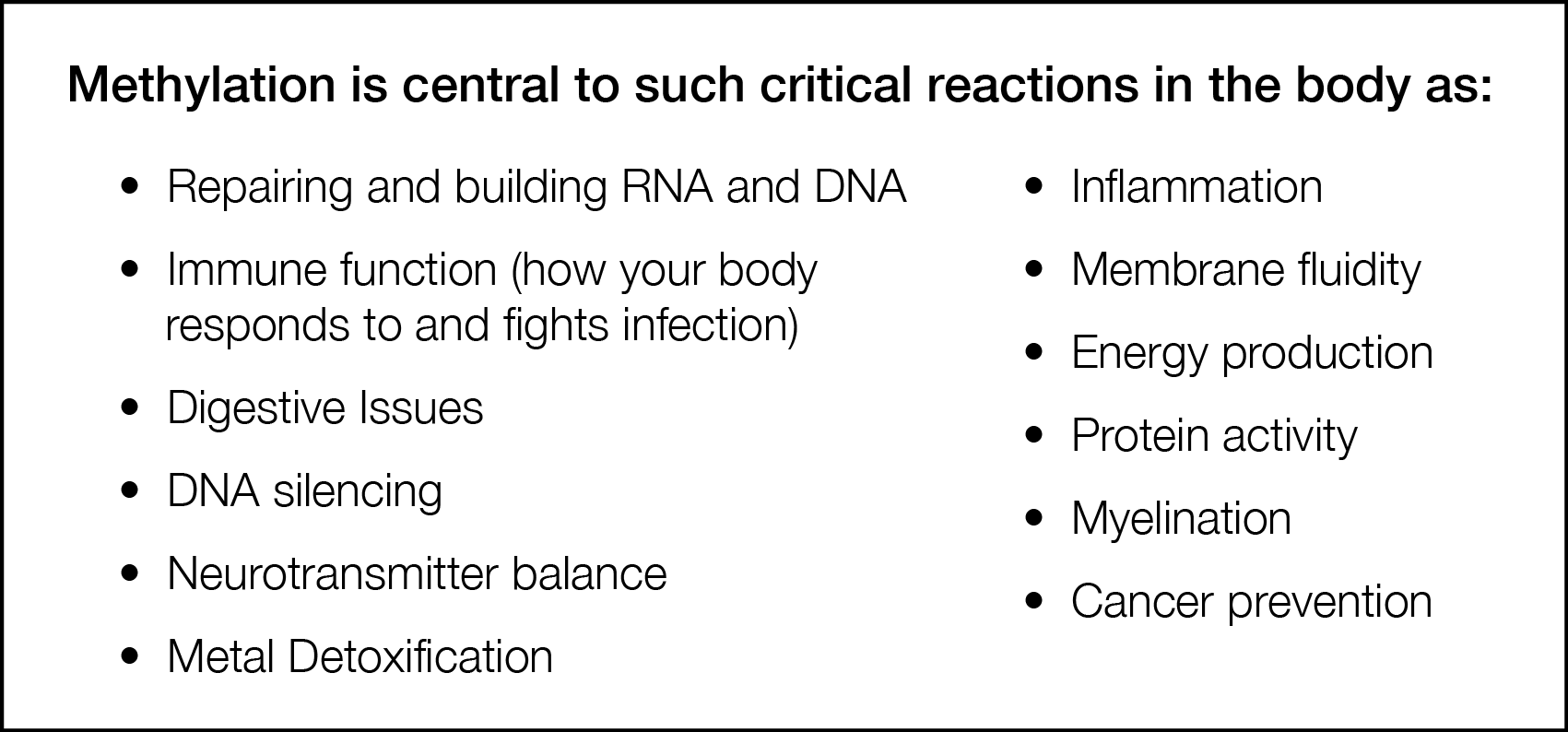
Methylation is involved in almost every reaction in your body. Aside from its critical editing function, without proper methylation, there is increased vulnerability to viruses, impaired attention span, and less efficient nerve transmission and a greater predisposition to cancer.
For an organism to live, it must create new cells as fast as cells die. This requires that the body make millions of cells every minute, relying on DNA and RNA synthesis. DNA carries the blueprint, or genetic coding, needed to build the components of living organisms. Every time your body needs to repair the gut lining, or create an immune cell to respond to an immune threat, or to heal when you have cut yourself, you need to synthesize new DNA. But without a functioning methylation cycle, your DNA is not going to replicate properly. Mutations in the methylation pathway can cripple the ability of the body to make the building blocks needed for new DNA and RNA synthesis. Very similar to DNA is RNA, which is crucial to building proteins, transferring the information carried by your DNA and regulating your genes. In fact, RNA is even more abundant in your body than DNA. This reduced capacity for new DNA and RNA synthesis means that any new cell synthesis is impaired. A reduced synthesis capacity due to methylation cycle mutations is a particular issue for cells that already have difficulties meeting their needs for DNA and RNA synthesis under normal conditions. Problems in the methylation pathway limit nucleotide building blocks that the brain and other organs need for repair and growth. Bone marrow cells, lymphocytes, erythrocytes and some brain cells cannot make some of the DNA and RNA bases that they need for synthesis. Intestinal mucosa cells cannot make enough building blocks to fulfill the body’s requirement for healthy gut. Stress increases the need for these building blocks to overcome negative effects of hormones released during stressful conditions. Cell repair after injury increases the need for nucleotide building blocks. The brain has the highest concentration of RNA in the body, and therefore has the highest requirement for RNA building blocks.
Universal lack of methylation and the inability to produce these building blocks for RNA synthesis also results in a situation where the body is lacking the required elements for specific genetic regulation. This regulation or silencing is a multistep process that involves RNA as well as methylation to be certain that only the desired genetic material is expressed. As described earlier, epigenetics is the mechanism used by the body to turn on and off genes. It is the editing system that gives the body a second chance to get around direct mutations in our DNA. Epigenetic modification of DNA occurs mainly on a very specific DNA building block called cytosine. Cytosine is one of the four DNA bases found in organisms, including humans. It is one of the DNA building blocks that are produced by optimal functioning of the Methylation Cycle. So, take a moment and think about this…Methylation Cycle function is needed to produce the building blocks for DNA that are in turn the recipients of methylation groups in order to turn on and off that same DNA. You can start to see how intimately related your DNA synthesis and function is with respect to the Methylation Cycle. Methylation of these cytosine bases is generally correlated with silencing of genes.
Methylation is important for turning on and off mammalian DNA. This is true for silencing viral DNA in the body as well as cellular DNA. There are sections of the DNA that are regulatory regions, requiring methylation such that they turn on and off the information portions as they should. During development DNA methylation patterns are established and are essential for normal development. During new cell synthesis these patterns are then replicated. When these regions do not have the correct amount of methyl groups bound to them it can prevent the information from being turned off, resulting in autoimmunity, aging and cancer.
It has been estimated that 70 to 80% of the cytosine’s found in particular patterns in the DNA are methylated in humans. The other 20% of these cytosine patterns that are not methylated are found in clusters known as “islands”. These nonmethylated islands are most often found in the region that turns on the gene. Thus methylation of cytosines creates two distinct regions in the DNA, “unmethylated islands” and “methylated cytosine pattern sites” that are distributed throughout the genome. These methylated regions tend to be located at mutational hot spots; one third of all single base mutations associated with cancer are at these sites.
Methylation of cytosine also helps to maintain the large amount of the non-utilized portion of the human DNA in an inert state as well as helping to silence harmful DNAs. If you are short on methyl groups due to methylation cycle mutations then you will have less methyl groups for preventing autoimmunity and silencing genomes. This is true for silencing viral genomes as well as for regulating your own DNA. The methylation process prevents the reading of inserted viral sequences. One of the consequences of loss of methylation function is that it could cause the potentially harmful expression of these inserted viral genes. Under-methylation in normally silent regions of the DNA can cause the expression of inserted viral genes.
Certain disease states occur as a result of increases in the length of specific repeat sections in the genome. These special “repeat regions” are prior to the information or coding region of the DNA. These trinucleotide repeats are involved in certain disorders such as Friedreich’s ataxia, Fragile X and Huntington’s disease. When there is insufficient methylation capacity (mutations in the pathway) there is often not enough methyl groups to bind to these repeat regions, so they are able to multiply. This results in very long repeat sections, much longer than they should be. Studies have shown that inhibition of DNA methylation resulted in a 1000 fold increase in these three base repeat sections. Therefore, decreased DNA methylation results in increases in trinucleotide repeats and increases the risks for these disorders. This creates a catch 22 as these long repeated sections then attract the limited methyl groups that are available. The consequent overmethylation of these repeat sections results in shutting off genes inappropriately.
Females contain two copies of the X chromosome. Silencing one of these two copies is essential for normal development. Methylation of the DNA is the mechanism by which the second X chromosome is silenced. The normally nonmethylated “islands” become methylated as part of this silencing process. A similar strategy is utilized to silence one of two copies of genes other than those on the X chromosome. In these cases the inactivated gene can be of either maternal or paternal origin. Loss of normal silencing as a result of decreased methylation contributes to a number of inherited diseases including Beckwith-Wiedemann, Prader-Willi and Angelman syndromes, among others.
The expression of many cellular genes is modulated by something called “histone acetylation” in addition to DNA methylation. Interestingly, methylation also plays a role in the histone acetylation process. Methylation also plays a pivotal role in establishing and maintaining an inactive state of a gene by rendering the chromatin structure inaccessible. Methylation therefore plays an important role in development, imprinting, X-chromosome inactivation and tissue-specific gene expression. Changes in DNA methylation profiles are common features of development and in a number of human diseases.
You can start to see how proper Methylation Cycle function would play a role in fetal development. It also plays a role in successful preconception supplementation to prevent miscarriages. Mutations in the MTHFR genes of the methylation pathway as well as mutations that lead to decreased B12 are risk factors for neural tube defects. Mutations in the methylation pathway, specifically certain MTR and MTRR SNPs, as well as elevated homocysteine are risk factors for having a child with Down’s syndrome.
It is important to consider methylation pathway mutations when looking at supplementing with folate during pregnancy. One way to understand this more easily is to think about the studies on folate and neural tube defects. Using folate during pregnancy helps to decrease the risk of neural tube defects. This is not changing the DNA but having a regulatory effect on the ability of the DNA to be expressed, known as epigenetics. Now, if folate can make a difference in DNA expression, but you have a mutation so that you cannot use folate, then taking folate may not do any good; it is almost as if you never supplemented at all. Running a nutrigenomic test to determine the form of folate that will bypass mutations in your folate pathway will enable you to supplement with the appropriate form of folate and should help to reduce the risk of neural tube defects in a similar way to the use of plain folate in the absence of mutations in this pathway. The genetics of the parent are reflected in the child. So that if a pregnant mother has mutations that make her unable to utilize plain folate, you should consider testing the infant for similar weaknesses in this pathway. The sooner that you know if and where a newborn’s genetic weaknesses reside in the methylation pathway the sooner you can start to supplement to bypass these mutations. Remember, by supplementing properly you should have the potential to bypass and compensate for the mutations. If this is commenced from day one you do not allow time for virus to build in the system (remember that methylation is necessary to silence virus). In addition, some of the mutations in the methylation cycle make it difficult to make new T cells which are a critical part of your immune system. This may make it easier for the immune system of infants to react to vaccines in the correct fashion. If the methylation cycle is working properly from day one it should help with myelination, immune regulation and the ability to make new DNA and RNA that is needed for growing cells.
In the methylation pathway, one crucial component for neurotransmitter balance is the component, S-adenosyl methionine, or SAMe (pronounced “sammy”). SAMe is the most active methyl donor in your body, bringing methyl groups to numerous chemical compounds in your body. It is a required participant in at least forty different critical reactions in the body. It also acts upon the neurotransmitters by changing them into other needed compounds. If we don’t have sufficient SAMe—or if SAMe can’t be recycled due to weaknesses in the methylation cycle, this can result in imbalances in our neurotransmitters, which in turn can impact mood, focus, sleep patterns, as well as a range of behaviors. The Methylation Cycle not only has to produce SAMe, it also has to recycle it. Once SAMe has given up its methyl groups to help create neurotransmitters, it is then “recycled”—that is, re-methylated. After SAMe has received its new methyl groups, it can perform its job all over again. Because of its essential role in reactions involving neurotransmitters, it’s not surprising that a lack of SAMe plays a role in neurodegenerative conditions. Due to Methylation Cycle weaknesses, some people can neither produce nor recycle SAMe. Furthermore, issues with the level of SAMe in the body became a larger problem as we age. Fortunately, we can supplement SAMe to bypass mutations and attain its many benefits.
Some specific reactions that involve SAMe include:
- Converting serotonin to melatonin which supports healthy sleep
- Glutathione synthesis which is critical for detoxification
- In the formation of myelin which surrounds and protects nerves
- To convert the neurotransmitter norepinephrine into epinephrine, (also known as adrenaline). Together norepinephrine and epinephrine regulate the fight-or-flight response and, along with dopamine, are critical for attention and focus.
- In the creation of CoQ10, creatine, and carnitine, compounds essential to the work of the mitochondria, the energy factories of our cells
Adequate levels of CoQ10 have also been identified as necessary nutrients to help prevent congestive heart failure. Clinically the supplement CoQ10 has been used in the treatment of angina, heart failure, prevention of reperfusion injury after coronary artery bypass and cardiomyopathy. The synthesis of CoQ10 in the body requires components of the Methylation Cycle; in particular it requires adequate levels of the compound SAMe (s adenosyl methionine) that is generated by the methylation cycle. Cholesterol lowering drugs (statin drugs) decrease the supply of CoQ10 in the body. It may be particularly important for individuals taking statin drugs to be aware of the methylation status in their body and replenish CoQ10. In addition, the relationship between elevated homocysteine levels, an increased risk of heart disease and the genetic risk associated with certain MTHFR mutations in the Methylation Cycle has been recognized for quite some time. Appropriate supplementation of the Methylation Cycle should be able to help compensate for this mutation.
The mitochondria are the energy producing organelles within each cell. Decreased mitochondrial energy has been implicated in chronic fatigue, fibromyalgia and mitochondrial disease. Coenzyme Q10 is also important for its role in energy production in the mitochondrial respiratory chain. Again, as mentioned above, Methylation Cycle function is necessary for the synthesis of CoQ10 in the body. Carnitine is another nutrient produced by the body that is involved in mitochondrial energy production. Mitochondria fatty acid oxidation is the main energy source for heart and skeletal muscle. Carnitine is also involved in the transport of these fatty acids into the mitochondrial matrix. As with CoQ10, the synthesis of carnitine by the body requires Methylation Cycle function. Synthesis of carnitine begins with methylation via the action of SAMe. Low muscle tone and extreme muscle weakness may in part be due to decreased mitochondrial energy as well as myelination problems due to reduced Methylation Cycle capacity. Methylation is also needed to produce creatine in the body. The methyl group for this reaction is again donated by SAMe. Creatine is a supplement taken by many weight lifters due to its role in muscle energy; creatine has also been reported to play a role in speech, language, attention span and ability to follow commands.
There is literature to suggest that ADD/ADHD can be helped by the addition of SAMe. SAMe is a critical intermediate in the Methylation Cycle. SAMe is also a methyl donor for reactions that involve dopamine, epinephrine and norepinephrine. Imbalances in this dopamine, epinephrine and norepinephrine have been implicated in ADD/ADHD. One can envision that Methylation Cycle function is needed to produce SAMe as a methyl donor for the dopamine/ nor epinephrine / epinephrine pathways to help to prevent ADD/ADHD. Impaired methylation results on a lack of components needed to generate feel good neurotransmitters like serotonin which regulates mood, emotion and appetite, as well as problems converting serotonin to melatonin so we can sleep at night. Many children with autism have difficulty sleeping as well as adults with chronic fatigue and fibromyalgia will frequently note sleep issues. Imbalances in methylation also affect dopamine levels as well as the dopamine receptor itself. Proper dopamine signaling requires that the dopamine receptor can move freely in the cell membrane. Methylation supports receptor activity by keeping the components of the cell membrane (phospholipids) more fluid. Imbalances in dopamine receptor signaling have been implicated in ADD/ADHD.
Myelin coating on nerves is important for proper neurotransmission. Myelin is a sheath that wraps around the neuronal wiring to insulate and facilitate faster transmission of electrical potentials. You can think of myelin as the coating around the electrical wires in your home. If those wires are not covered then even a single drop of water can cause the wire to short out. So too, your nerves are more vulnerable to assaults if they are not coated in myelin. Without adequate methylation, the nerves cannot myelinate in the first place, nor remyelinate after insults such as viral infection or heavy metal toxicity. In addition, methylation helps to stabilize myelin against degradation. Proper levels of methylation are also directly related to the body’s ability to both myelinate nerves and to prune nerves. A secondary effect of a lack of methylation and hence decreased myelination is inadequate pruning of nerves. Pruning helps to prevent excessive wiring of unused neural connections and reduces the synaptic density. Without adequate pruning the brain cell connections are misdirected and proliferate into dense, bunched thickets. All of these changes, when they occur in utero or in very young children, can alter brain development and can also set up metabolic changes that cause ongoing compromise of brain function. These metabolically caused changes in brain function may, however, be mitigated if the underlying nutrigenomic weaknesses that are causing these changes are identified and supplemented nutritionally.
Methylation of cytosine is generally correlated with silencing of genes. One difference between bacterial and human genomes is that bacterial genes are not methylated at specific cytosine regions. Research has shown that when mammalian genes are not methylated at these regions it can trick the immune system into reacting against itself and causing autoimmunity. New cell synthesis is needed in order for certain types of immune cells to expand and respond properly to an immune assault. These same immune cells are also involved in controlling the overall immune response, keeping it in balance. If there are Methylation Cycle problems or mutations, you may have trouble making the bases that are needed for new DNA synthesis. If you cannot make new DNA, then you cannot make these specialized immune cells and as a result you may lack immune system regulatory cells.
The immune system has the B cell “arm” that makes antibodies, known as humoral immunity and the T cell “arm” known as cellular immunity. If you are having trouble making new T cells, then the immune response may become more heavily weighted in the direction of B cells. The B cells have the ability to respond by making antibody, or auto antibody rather than making the range of T cells that regulate as well as fight infections. B cell clones expand and then are available for the future. So there is a somewhat greater need for new DNA synthesis for the T cell response than for the B cell response. Individuals with Methylation Cycle mutations are more at risk as they will have problems making regulatory T cells that help the body to control the B cells and prevent autoimmune antibodies. Auto antibodies can occur as a result of imbalances in immune regulation. If you are not making adequate T cells (methylation pathway mutations) then you may lack regulatory T cells and can end up with auto antibodies.
Methylation also plays a role in the ability of the immune system to recognize foreign bodies or antigens that it needs to respond to. Research has shown that methylation is decreased in humans with auto immune conditions. Impaired methylation of T cells may be involved in the production of auto antibodies. Studies from patients with systemic lupus erythrematosis (SLE) have shown that their T cells are undemethylated. Impaired methylation of T cells may also be involved in the production of auto antibodies. Studies from patients with systemic lupus erythrematosis (SLE) have shown that their T cells are undermethylated. As proper methylation function is restored, it should help in regaining immune function regulation. In several cases I have seen the level of auto antibodies decline after proper Methylation Cycle supplementation.
Methylation of DNA is also used to regulate immune cells. Immune receptor DNA is initially in the ”OFF” state and is maintained that way until the immune cells need to differentiate. At that time the methyl groups are removed from the DNA in a highly regulated fashion.
Studies show that decreased methylation of cytosine regions in these immune genes may influence the balance of immune inflammatory cells known as TH1 and TH2. The effect of methyl groups on the TH1/TH2 balance may be another mechanism by which decreased methylation may increase allergies. There are two sets of T helper cells in the immune system, TH1 and TH2 cells. While TH1 cells are involved in cell mediated immune responses and toning down or regulating TH2 activity, the TH2 cells have been associated with humoral or B cell mediated responses and allergic responses. TH2 cells trigger the activation and recruitment of IgE antibody producing B cells, mast cells and eosinophils that are involved in allergic inflammation. In addition, when methylation is impaired it can lead to abnormally high levels of histamine. High levels of histamine are causative factors in allergic reactions. Optimal methylation function is needed to break down histamine so that it does not build up in the body, in addition to the effect of methylation on histamine levels.
The levels of various metabolites of the methylation pathway are important for protection from side effects of anesthesia. As early as 1942 it was recognized that the addition of methionine is preventative for side effects from the use of chloroform. Methionine affords protection from liver injury as a result of chloroform anesthesia. Methionine also protects against effects of nitrous oxide anesthesia. Nitrous oxide disrupts the activity of MTR, a central enzyme in the Methylation Cycle. Again, preloading with methionine appears to accelerate recovery and reduce side effects associated with this form of anesthesia. The neurological deterioration and death of an infant boy has been reported who had been anesthetized twice within a short time with the anesthetic nitrous oxide. Postmortem studies determined that this child had a deficiency of the MTHFR which is a principle enzyme in the Methylation Cycle.
The relationship between environmental toxins and DNA methylation is extremely complex. Environmental toxins can impact the extent to which DNA is methylated. “A hypothesis that is gaining ground is that environmental factors achieve their effect by altering the epigenetic profile of the cell” conversely epigenetics may “thus explain why certain individuals are more susceptible to certain environmental toxins” (Traynor, Neuron). Furthermore, methylation is also required to clear environmental toxins from the body. This process involves conjugating methyl groups to the toxins prior to removal. Most of the methyl groups that are used for detoxification are donated by SAMe. Elimination of inorganic arsenic from the body requires methylation. After methylation arsenic can be eliminated from the body in the urine. Differences in methylation may also account for susceptibility of different tissues to cadmium toxicity. In animal studies, methylation was necessary to induce metallothionein activity that was required for cadmium excretion. The methylation process is also the major means of detoxifying excess selenium in the body. Nutritional support for the methylation pathway was able to prevent strychnine induced seizures and death in animal models, as well as to be protective against carbon tetrachloride induced toxicity. Supplementation was also able to prevent ethanol induced decreases in Methylation Cycle function. Compounding the situation, environmental toxins are also able to have a negative impact on methylation. In experimental models, exposure of animals to environmental toxins during development appears to alter the pattern of DNA methylation. This change in DNA methylation is then maintained and carried forward to future generations of offspring. The heavy metals, arsenic, nickel and chromium are able to cause over methylation of DNA. This can result in turning “OFF” of important regulatory genes such as tumor suppressor genes. In addition, other environmental contaminants such as polycyclic aromatic hydrocarbons (PAH) and benzo(a)pyrene diol epoxide (BPDE) are able to bind to methylated cytosine regions of the DNA. Cadmium also inhibits the methylation of phospholipids, interfering with cellular membrane functions.
Undermethylation of the entire genome is referred to as global hypomethylation. Global hypomethylation when paired with over methylation of highly select repeated regions of the gene is associated with both aging and cancer.
Intermediates of the methylation pathway are known to decrease with age along with a decline in Methylation Cycle function. DNA methylation is also known to decrease with aging. Age related decreases in methylation can lead to decreased methylation of T cells which may in part explain changes in immune function with age. Age related decreases in methylation can result in increased levels of homocysteine, increasing the risk of arthritis, cancer depression and heart disease. This would suggest that increasing the body’s level of methylation through supplementation may extend a healthy life span. Both undermethylation of tumor causing genes (no turn OFF) and overmethylation of tumor suppressing genes (turned OFF) have been well characterized as contributing factors to cancer.
Methylation is used to inactivate excess levels of endogenous products that may be harmful to the body. For instance, excess estrogen is inactivated by methylation, with SAMe donating a methyl group for this process. The inability to inactivate excess estrogen has been linked to an increased susceptibility to hormone sensitive cancers. Epidemiologic and mechanistic evidence suggests mutations in the Methylation Cycle are involved in colorectal neoplasia. Specifically, the role of certain MTHFR mutations, MTR and MTRR mutations have been implicated in colorectal cancer.
The overwhelming impact of Methylation Cycle mutations is exemplified by the article in Science News which reported that although identical twins have the identical DNA they often have differences in a number of traits including disease susceptibility. This study suggests that as twins go through life the environmental influences to which they are exposed affects which genes are actually turned on or off. Methyl groups can attach to the DNA in a similar way that charms attach to a charm bracelet. This modification of the DNA is what I have already described as epigenetic regulation. The combination of environmentally determined addition of these “charms” to the bracelet of DNA, combined with inherited DNA changes or mutations lead to an individual’s susceptibility to disease. According to the scientist who headed this study, Dr. Manuel Estseller, “My belief is that people are 50 percent genetics and 50 percent environment”.
This statement should give us some understanding as to why mutations in the Methylation Cycle can be so devastating. Mutations in the Methylation Cycle affect the 50% that represents genetic susceptibility; this would be analogous to defects in the links of the chain of our charm bracelet. In addition, because methylation is also necessary for the epigenetic modification of the DNA, methylation also affects the environmental 50%.
If we take the analogy a step further to really understand the global impact of defects in this pathway we can view genetically inherited mutations in the methylation pathway as causing problems in the links of the bracelet and environmental effects creating a problem with the ability to put charms on the bracelet of DNA. Problems in the Methylation Cycle therefore can affect 100% of our susceptibility to disease. This is why it is critical for health reasons to understand where our weaknesses in this pathway reside and then supplement appropriately to bypass these mutations.
A second study that has also addressed the nature versus nurture question used animal models to look at this issue. Researchers were able to show that the adult response to stressful situations was heavily influenced by the interactions these same animals had as pups with their mothers at birth. Those pups with higher levels of care showed differences in the methylation patterns of stress related genes when compared with pups in the lower care test group. Dr. Szyf from the team at McGill University that conducted the study has stated that their study results “…have bridged the gap, nurture is nature”.
This work does suggest that the bridge between “nature and nurture” is the ability of nurturing to influence DNA methylation. However, nurture alone cannot be the answer. According to this study nurturing can influence epigenetic modification of DNA, so nurturing can affect the number of “charms on the bracelet”. However if there are genetic mutations in the DNA sequence itself, the actual “links of the bracelet” this will also affect the overall methylation capacity in the body. Without the mechanisms to produce the methyl groups, all of the nurturing in the world will not be able to overcome the lack in the production capacity for methylation. In other words if the body cannot produce the charms for the bracelet it becomes a moot point how easily you are able to attach them to the bracelet. Nutrigenomic support to bypass these mutations is one mechanism to address the weaknesses in the DNA that would result in reduced capacity in this pathway.
![]()